The application of First Law of Thermodynamics process led to the establishment of a new property named internal energy (U), the application of the second law to a process leads to the establishment of another new property names as entropy (S).
Entropy is defined as follows
Δ
S
=
Q
T
rev
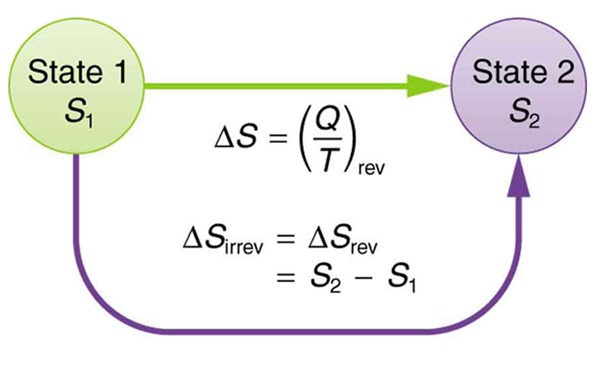
For a reversible thermodynamic process between two states, from state 1 to state 2 in an open system, the change in entropy is given by

It is found by applying Clausius inequality that for for an irreversible process from state 1 to state 2, the change in entropy is given by

No comments:
Post a Comment